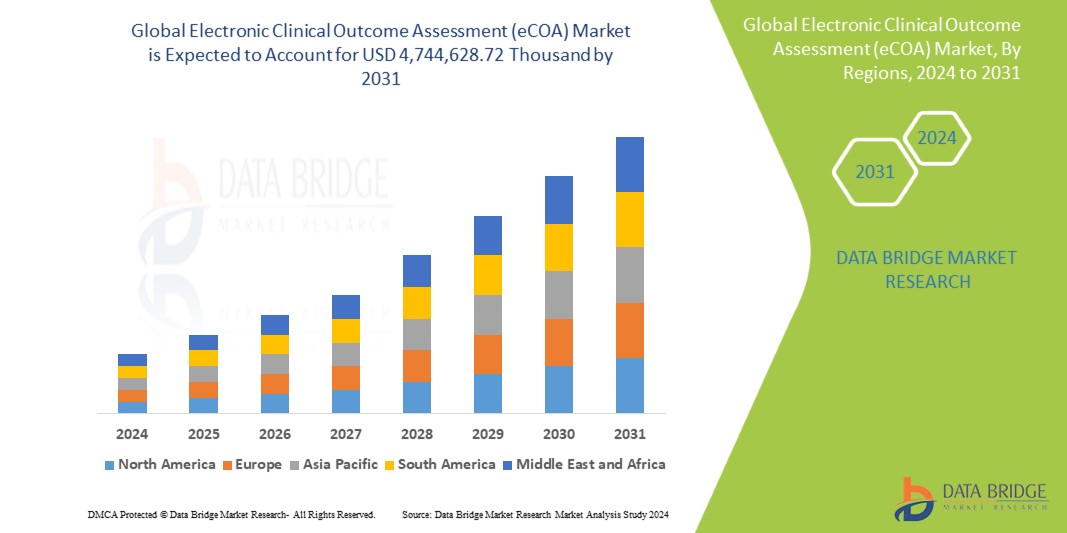
Introduction
The Electronic Clinical Outcome Assessment (eCOA) market is a critical component of the modern healthcare and clinical research landscape. eCOA involves the digital collection of patient data related to health outcomes through various electronic means, including smartphones, tablets, computers, and specialized devices. This method improves the accuracy, efficiency, and reliability of data collection in clinical trials and real-world studies. The eCOA market is rapidly growing as healthcare providers and researchers increasingly adopt digital solutions to enhance the quality and speed of clinical research. This post delves into the evolution of the eCOA market, current trends, growth drivers, market scope, market size, and a country-level analysis.
The Evolution of the eCOA Market
The concept of eCOA emerged as a response to the limitations of traditional paper-based data collection methods in clinical trials. Historically, clinical outcome assessments were conducted using paper forms, which were prone to errors, delays, and data loss. As technology advanced, the need for more efficient, accurate, and patient-friendly data collection methods became apparent. This led to the development of electronic data capture (EDC) systems, which eventually evolved into more sophisticated eCOA solutions.
The early 2000s marked the beginning of eCOA adoption, with the initial focus on electronic patient-reported outcomes (ePROs). These digital tools allowed patients to directly input their health-related information through electronic devices, reducing the risk of transcription errors and improving data quality. Over time, eCOA expanded to include clinician-reported outcomes (ClinROs), observer-reported outcomes (ObsROs), and performance outcomes (PerfOs), making it a comprehensive solution for clinical data collection.
The evolution of eCOA has been driven by advancements in mobile technology, cloud computing, and data analytics. The widespread availability of smartphones and tablets has made it easier for patients to participate in clinical trials from the comfort of their homes, while cloud-based platforms have enabled real-time data collection and analysis. As a result, eCOA has become an essential tool in clinical trials, particularly in decentralized and remote trials, which have gained popularity in recent years.
Market Trends and Factors Driving Growth
Several key trends are shaping the eCOA market today. One of the most significant trends is the increasing adoption of decentralized clinical trials (DCTs). DCTs, which allow for remote patient participation and data collection, have gained traction in response to the challenges posed by the COVID-19 pandemic. eCOA solutions are a critical component of DCTs, enabling seamless and accurate data collection from patients regardless of their location. This trend is expected to continue as the clinical research industry embraces more flexible and patient-centric trial designs.
Another important trend is the growing emphasis on patient-centricity in clinical research. There is a growing recognition of the importance of involving patients more actively in the clinical trial process, and eCOA tools are instrumental in achieving this goal. By providing patients with easy-to-use electronic platforms for reporting their health outcomes, eCOA enhances patient engagement, compliance, and satisfaction. This shift towards patient-centric trials is driving the demand for eCOA solutions.
The integration of eCOA with other digital health technologies, such as wearable devices and telemedicine platforms, is also contributing to market growth. Wearable devices, for example, can continuously monitor and transmit patient health data, which can be seamlessly integrated into eCOA platforms for real-time analysis. This convergence of technologies is creating new opportunities for more comprehensive and accurate data collection in clinical trials.
Factors driving the growth of the eCOA market include the increasing complexity of clinical trials, the need for more efficient and accurate data collection methods, and the rising demand for real-time data analysis. As clinical trials become more complex, with multiple endpoints and diverse patient populations, the need for reliable and scalable data collection solutions becomes more critical. eCOA addresses these challenges by providing a flexible, scalable, and patient-friendly solution for capturing clinical outcome data.
Regulatory support for eCOA is another key factor driving market growth. Regulatory agencies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have recognized the benefits of eCOA and have provided guidelines for its use in clinical trials. These guidelines have helped to standardize eCOA practices and have encouraged its adoption across the industry.
Market Scope and Market Size
The scope of the eCOA market is broad, encompassing a wide range of applications in clinical trials and healthcare. eCOA solutions are used in various therapeutic areas, including oncology, cardiology, neurology, and rare diseases. These solutions are applied in different types of clinical trials, from early-phase studies to large-scale, late-phase trials, as well as in real-world evidence (RWE) studies. eCOA is also used in post-marketing surveillance and patient registries, where ongoing monitoring of patient outcomes is required.
The eCOA market includes a diverse range of products and services, from standalone ePRO tools to comprehensive eCOA platforms that integrate multiple types of outcome assessments. These solutions are delivered through various deployment models, including on-premise, cloud-based, and hybrid models. The cloud-based model is particularly popular due to its scalability, flexibility, and ease of access, making it ideal for decentralized and global clinical trials.
In terms of market size, the global eCOA market has been experiencing robust growth and is expected to continue expanding in the coming years. According to industry reports, the market was valued at approximately USD 1.5 billion in 2020 and is projected to reach around USD 3.5 billion by 2027, growing at a compound annual growth rate (CAGR) of 12.8%. This growth is driven by the increasing adoption of digital solutions in clinical trials, the rise of decentralized trials, and the growing emphasis on patient-centric research.
Country-Level Analysis
The adoption of eCOA varies across different regions and countries, influenced by factors such as regulatory frameworks, technological infrastructure, and the level of digitization in clinical research. North America, particularly the United States, is a leading market for eCOA. The region’s advanced technological infrastructure, coupled with strong regulatory support for digital health solutions, has created a favorable environment for the adoption of eCOA in clinical trials.
In Europe, countries like the United Kingdom, Germany, and France are at the forefront of eCOA adoption. The European Medicines Agency (EMA) has provided clear guidelines on the use of eCOA in clinical trials, encouraging its adoption across the region. Additionally, Europe’s focus on patient-centric research and the increasing digitization of healthcare are driving the growth of the eCOA market in the region.
The Asia-Pacific region is expected to witness the fastest growth in the eCOA market, driven by the rapid expansion of clinical research activities in countries like China, India, Japan, and South Korea. These countries are increasingly adopting digital health technologies, including eCOA, to enhance the efficiency and quality of clinical trials. The growing pharmaceutical and biotechnology industries in the region are also contributing to the demand for eCOA solutions.
Latin America and the Middle East & Africa regions are emerging markets for eCOA. In Latin America, countries like Brazil and Mexico are seeing increasing investments in clinical research and digital health infrastructure, driven by government initiatives to improve healthcare quality and access. The Middle East, particularly the Gulf states, is also investing in eCOA as part of their broader efforts to modernize healthcare systems and attract international clinical trials.
Conclusion
The eCOA market is poised for significant growth as clinical research continues to evolve towards more digital, patient-centric, and data-driven approaches. The market is being driven by the increasing adoption of decentralized clinical trials, the integration of eCOA with other digital health technologies, and the growing emphasis on real-time data analysis. With diverse applications and significant regional variations, the eCOA market presents numerous opportunities for innovation, investment, and growth in the coming years. As the healthcare and clinical research industries continue to embrace digital transformation, eCOA will play a crucial role in shaping the future of clinical trials, improving the accuracy, efficiency, and patient experience in the process.
Get More Detail: https://www.databridgemarketresearch.com/reports/global-electronic-clinical-outcome-assessment-ecoa-market